Effectiveness
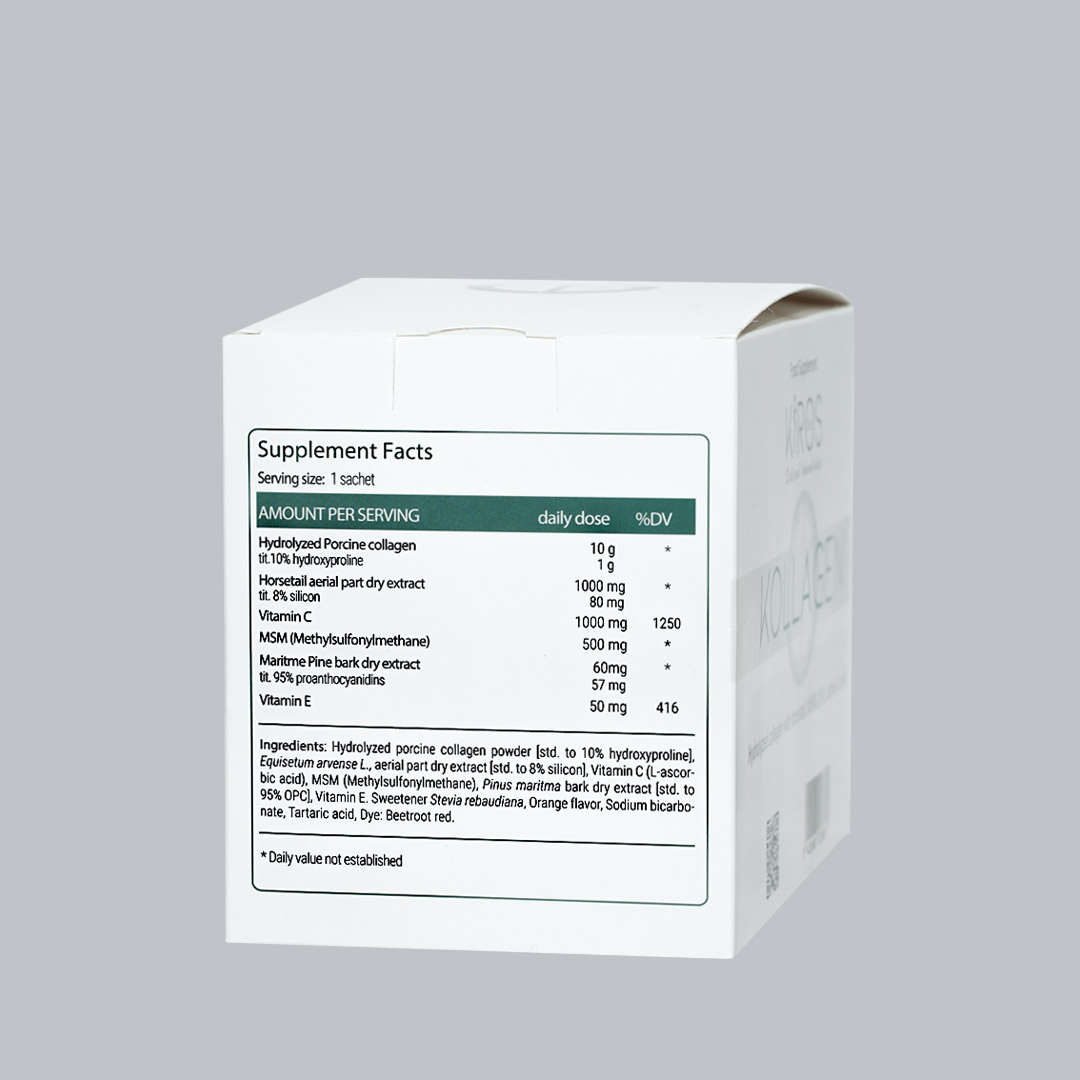
Kiros Kollagen. Is a nutraceutical composed of Hydrolized suine Collagen powder, Vitamin C, Equisetum arvense extract (tit. 7% in silicon), MSM, Maritime pine extract (95% OPC) and Vitamin E. Useful in all cases of reduced dietary intake or increased need for useful components to improve collagen synthesis in the body.
Assumption
1 sachet per day to be dissolved in approximately 200 cc of water to be taken during meals or between meals or according to the instructions of the doctor or nutritionist.
It is preferable to mix Orange Collagen with a sachet of alkaline and 1-2 squeezed lemons.
Contest
Collagen is one of the most important proteins in vertebrates and represents one third of the total proteins of the human body, where it plays a fundamental role in the structure and functionality of organs and tissues, such as skin, cartilage, muscle tissue. It has been established that the collagen fibers are damaged over time: at the age of 25 cells begin to lose the ability to synthesize collagen, whose production decreases by 1.5% per year. This process is accentuated over 45 years old and when approaching 60, the production of collagen has decreased by over 35%, losing thickness and strength. In addition, there are other factors that intensify the loss of collagen: overuse (intense physical activity), trauma, menopause, overweight, hormonal therapies, incorrect diets, oncological treatments, intense and continuous exposure to sunlight or to particular environmental conditions (pollutants -smoke -stress). Collagen supplements have been claimed to promote brain, heart and gut health, as well as to help control weight and keep hair and nails healthy.
Characteristics of the components
Collagen is the most plentiful fibrillary protein of the body that conforms the conjunctive and connective tissues in the human body, essentially skin, joints, and bones. It holds all the living tissues together and ensures the integrity, elasticity, regeneration of the skin, cartilages and bones and maintenance of tonicity of the skin thanks to the serum proteins, the hydroxyproline, cystine and silicon amino acids. Collagen accounts for 80% of our connective tissues and 30% of proteins in our body. There are 28 different types of collagen in the literature depending on the tissue in which it is found: type I is more expressed in the skin, tendons and bones; cartilage is rich in type II. What changes is only the three-dimensional organization that collagen takes in space, influenced by the “cross-linking” induced by a specific amino acid: hydroxyproline. This is the reason why we find it elastic in the skin, hard and consistent in nails, filamentous and flexible in the fibrils of the bone. The human organism does not absorb collagen as such, but it is digested through the action of the gastric enzymes, in the constituent components, represented by more or less long chains of amino acids; it is superfluous for exogenous collagen, to speak of “different types”, since the protein must necessarily be decomposed into fragments in order to “enter” the body and thus performs its precious physiological properties: the variation of the amino acid sequence of collagen, whatever it is, does not reflect changes in its physiological properties, but only a different conformational orientation in space. The daily integration of collagen should amount to about 1 gram per 10 kg of body mass1.
Hydrolized Pork Collagen is produced from fresh skin or food grade gelatin products. Is an effective food aid for the regeneration and protection of collagen, provides 16% of essential amino acids, 60% of conditionally essential amino acids, contains glycine, arginine and methionine, the 3 amino acids precursors of synthesis of creatine (regenerating ATP, the source of muscular energy). In its hydrolyzed form, porcine collagen is characterized by a high food absorption: bioavailability studies show that the intestinal absorption of the pork hydrolyzed collagen ingested rises to 82% in the first 6 hours after ingestion and to 95% in 12 hours.
Silicon from Equisetum arvense is a structural element of connective tissue and enters the constitution of the main macromolecules such as elastin, collagen, proteoglycans and glycoproteins, promoting their regeneration. The silicon content is closely related to the optimal skin conditions, such as the rate of hydration, elasticity, the absence of wrinkles and expression lines, the ability to heal and regenerate. It also acts as a metabolic protector, acting at different levels: it opposes the lipid peroxidation responsible for the liberation of free radicals, it counteracts the cross-linking and non-enzymatic glycosylation of the connective tissue proteins that cause stiffness and sclerosis, it regulates and stimulates the mitosis of fibroblasts and for this property, it plays an essential role in the process of dermal and epidermal cells regeneration, and lastly it is a cofactor of the elastin synthesis. Collagen synthesis is silicon-dependent, which influences the hydroxylation process of proline in hydroxyproline.
MSM (Methylsulfonylmethane) is a biologically active form of organic sulphur with anti-inflammatory, antioxidant and pain-relieving properties. It restores the formation of cross-links in collagen, preserving its elasticity, and is considered a synergistic element in stimulating collagen synthesis for the amino acids cystine, glycine, hydroxyproline, ornithine and silicon. MSM is a potent antioxidant, which can have positive effects on joints, help protect cells from oxidative stress and has a positive impact on the health of the skin and subcutaneous tissue.
Vitamin C: essential for the formation of collagen, it helps to maintain the integrity of substances of mesenchymal origin (connective tissue, osteoid tissue and dentin). It is a potent reducing agent which in the body is oxidized and reduced, functioning as a cellular redox system. In the absence of vitamin C, cells cannot hydrogenate proline in y positions, resulting in an instability of the collagen leading to scurvy.
Maritime pine extract. Collagen is degraded by proteolytic enzymes, called metalloproteinases. They break the long protein chains that make up collagen, forming shorter fragments with no mechanical properties. MMPs are a family of molecules similar in chemical structure, with different specificities for a wide variety of substrates, capable of degrading all the components of the extracellular matrix (collagen, elastin, laminins, proteoglycans). The MMPs most involved with skin aging processes are MMP1, which begins the degradation of type I and III collagen; MMP9, which operates a further fragmentation into smaller peptides; while MMP2 attacks type IV collagen, contributing to the formation of wrinkles. In young skin that is not exposed to sunlight, the synthesis of collagen by fibroblasts and its degradation by MMPs are in constant equilibrium, so as to guarantee the physiological replacement of structural macromolecules, keeping their content and functionality unchanged, and preserving the integrity of the skin. With increasing age there is a progressive imbalance of this equilibrium and there is a gradual reduction in the synthesis of collagen precursor peptides, associated with an increase in the degradation of mature collagen. The consequence of this degenerative phenomenon is a general disorganization of the collagen fibers, which partially lose their supporting role, causing a partial atrophy of the extracellular matrix, which becomes soft and lacking in tone. The maritime pine bark has excellent radical scavenging properties2. The extract act as inhibitor of metalloproteinases, resulting in proteolytic tissue damage or remodeling in pathophysiological conditions.
Studies
Collagen and cellulite
A clinical trial has been investigating the efficacy of collagen peptides on the cellulite treatment of normal and overweight women. In total, 105 women aged 24-50 years old with moderate cellulite were randomized to orally receive a daily dosage of 2.5 g bcp or a placebo over 6 months. The degree of cellulite was evaluated before starting the treatment and after 3 and 6 months of intake. In addition, skin waviness, dermal density, and the length of subcutaneous borderline were assessed. Collagen treatment led to a statistically significant decrease in the degree of cellulite and a reduced skin waviness on thighs in normal weight women. Moreover, dermal density was significantly improved as well as the subcutaneous borderline, indicating cellulite improvement. The efficacy of treatment was also confirmed in overweight women. Based on this study, it can be concluded that a long-term therapy with orally administered collagen leads to an improvement of cellulite and has a positive impact on skin health3.
Collagen improve bone mineral density and bone markers in postmenopausal women
These data demonstrate that the intake of collagen increased bone mineral density in postmenopausal women with primary, age-related reduction of bone mineral density. In addition, collagen supplementation was associated with a favorable shift in bone markers, indicating increased bone formation and reduced bone degradation4.
Collagen and sport
In a recent study, Zdzieblik et al.5 have investigated the effect of post-exercise protein supplementation with collagen peptides on muscle mass and muscle function during a 3-month resistance training programme. Subjects in the collagen-supplemented group showed a higher increase in Fat Free Mass and muscle strength as well as a higher reduction in Fat Mass.
An explanation for the observed effects could be that collagen is rich in arginine and glycine, both known to be important substrates for the synthesis of creatine in the human body. Creatine supplementation has been shown to improve both muscle mass and muscular function in some but not all studies6,7.
In addition, collagen peptides have shown to positively influence microcirculation that might cause an additional beneficial effect in promoting muscle growth compared with other protein sources.
Can be useful for
Skin
Your skin, Collagen is the major component and It plays a role in strengthening skin, plus may benefit elasticity and hydration8.
Increase skin hydration and reduce wrinkles and dryness9
Increase in skin elasticity10
Stimulate body production of collagen on its own8.
Promote the production of other proteins that help structure your skin, including elastin and fibrillin8,11
Maintain the integrity of cartilage
Joint Pain
Prevent degenerative joint disorders such as osteoarthritis12
Inhibit the bone breakdown that leads to osteoporosis13,14
Muscle Mass
Keep muscles strong and functioning properly15
Increased muscle growth and strength in people with age-related muscle mass loss5
Health
Help reduce the risk of heart-related conditions, providing structure to arteries and may help reduce the risk factors associated with heart conditions such as atherosclerosis
Stimulate your hair and nails to grow longer16
May promote weight loss and a faster metabolism.
Literature cited
- Khan, I. M., Gilbert, S. J., Singhrao, S. K., Duance, V. C., & Archer, C. W. (2008). Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater, 16(2008), 26-39.
- Grimm, T., Schäfer, A., & Högger, P. (2004). Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radical Biology and Medicine, 36(6), 811-822.
- Schunck, M., Zague, V., Oesser, S., & Proksch, E. (2015). Dietary supplementation with specific collagen peptides has a body mass index-dependent beneficial effect on cellulite morphology. Journal of medicinal food, 18(12), 1340-1348.
- Guillerminet, F., Beaupied, H., Fabien-Soulé, V., Tomé, D., Benhamou, C. L., Roux, C., & Blais, A. (2010). Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study. Bone, 46(3), 827-834.
- Zdzieblik, D., Oesser, S., Baumstark, M. W., Gollhofer, A., & König, D. (2015). Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. British Journal of Nutrition, 114(8), 1237-1245.
- Chilibeck, P. D., Chrusch, M. J., Chad, K. E., Davison, K. S., & Burke, D. G. (2005). Creatine monohydrate and resistance training increase bone mineral content and density in older men. Journal of Nutrition Health and Aging, 9(5), 352.
- Maughan, R. J., King, D. S., & Lea, T. (2004). Dietary supplements. Journal of sports sciences, 22(1), 95-113.
- Ganceviciene, R., Liakou, A. I., Theodoridis, A., Makrantonaki, E., & Zouboulis, C. C. (2012). Skin anti-aging strategies. Dermato-endocrinology, 4(3), 308-319.
- Proksch, E., Schunck, M., Zague, V., Segger, D., Degwert, J., & Oesser, S. (2014). Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin pharmacology and physiology, 27(3), 113-119.
- Proksch, E., Segger, D., Degwert, J., Schunck, M., Zague, V., & Oesser, S. (2014). Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin pharmacology and physiology, 27(1), 47-55.
- Borumand, M., & Sibilla, S. (2015). Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. Journal of Medical Nutrition and Nutraceuticals, 4(1), 47
- Porfírio, E., & Fanaro, G. B. (2016). Collagen supplementation as a complementary therapy for the prevention and treatment of osteoporosis and osteoarthritis: a systematic review. Revista Brasileira de Geriatria e Gerontologia, 19(1), 153-164.
- Moskowitz, R. W. (2000, October). Role of collagen hydrolysate in bone and joint disease. In Seminars in arthritis and rheumatism(Vol. 30, No. 2, pp. 87-99). WB Saunders.
- Elam, M. L., Johnson, S. A., Hooshmand, S., Feresin, R. G., Payton, M. E., Gu, J., & Arjmandi, B. H. (2015). A calcium-collagen chelate dietary supplement attenuates bone loss in postmenopausal women with osteopenia: a randomized controlled trial. Journal of medicinal food, 18(3), 324-331.
- Gillies, A. R., & Lieber, R. L. (2011). Structure and function of the skeletal muscle extracellular matrix. Muscle & nerve, 44(3), 318-331.
- Hexsel, D., Zague, V., Schunck, M., Siega, C., Camozzato, F. O., & Oesser, S. (2017). Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. Journal of cosmetic dermatology, 16(4), 520-526.